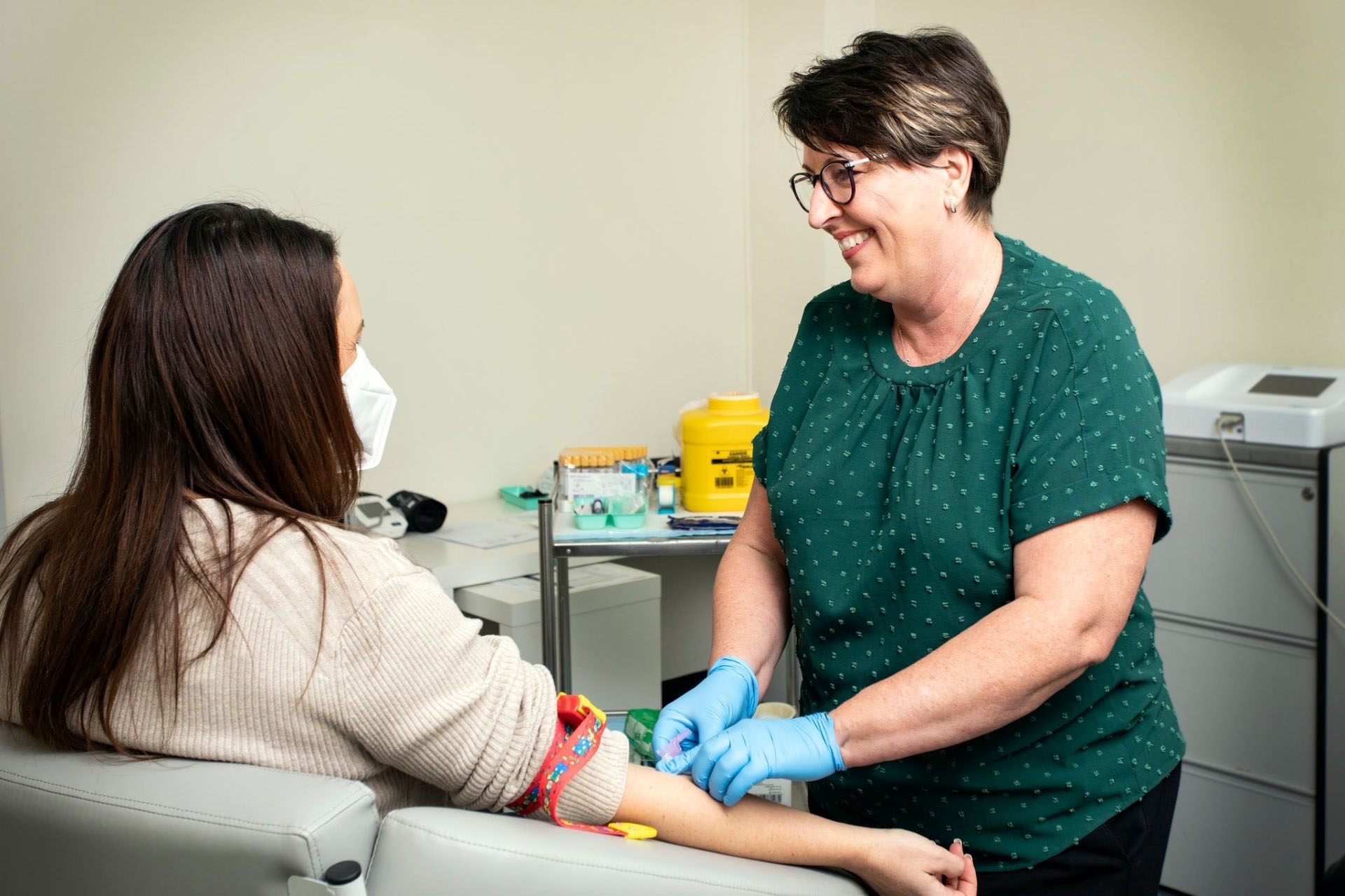
Gold standard choice
CRSA provides compassionate, state-of-the-art cancer care by improving access for patients in South Australia to novel cancer therapies. Clinical trials have been demonstrated internationally to be the gold standard choice of treatment for patients diagnosed with cancer.
We work alongside specialist cancer surgeons, radiation oncologists and all major medical specialties, to ensure each patient’s cancer care is optimised, individualised, and of the highest standard.
Why CRSA?
Expertise
- CRSA was created by a team of medical oncologists who practice at a number of private and public hospitals in Adelaide and include members from AOAH and ICON.
Access
- CRSA is located in a purpose-built facility overlooking the South Parklands, alongside St Andrew’s Hospital in the CBD.
- This location allows for convenient access to other specialists and healthcare options.
Advanced
- Efficient, fast delivery of trials and access for all to ‘gold standard’ cancer treatment.
- Ethics-driven practice committed to upholding patient and partner confidentiality.
Gold Standard
- Clinical trials often provide novel therapies and have been demonstrated internationally to be the gold standard choice of treatment.
Your treatment journey
A cancer diagnosis is life-changing and in your treatment journey it is important to feel that your wellbeing is always being considered.
At CRSA, we provide a calm and welcoming environment where everything you and your family need is close to hand.
Support CRSA

Support CRSA.
CRSA accepts donations (non-tax deductable) with funds going towards providing improved patient care and amenities.
Email: admin@crsa.au